Phosphate Crystal Precipitation and Pipe Clogging
WaterFX won’t clog your pipes or pumps.
Removing phosphorus from wastewater using conventional iron (or aluminum) based coagulants creates crystals that often redissolve and can then reprecipitate along pipe walls and pump impellers. These precipitated crystals then form a base for even more precipitation until the crystals clog pipes and pumps. This clogging causes operational downtime and requires considerable manpower to remove the obstructions or replace the impacted equipment. Looking at crystal solubility and precipitation mechanisms provides insight into how iron-phosphate crystals, called Vivianite, clog equipment – and why WaterFX is a better solution.
Vivianite’s solubility and propensity for wall growth leads to clogs.
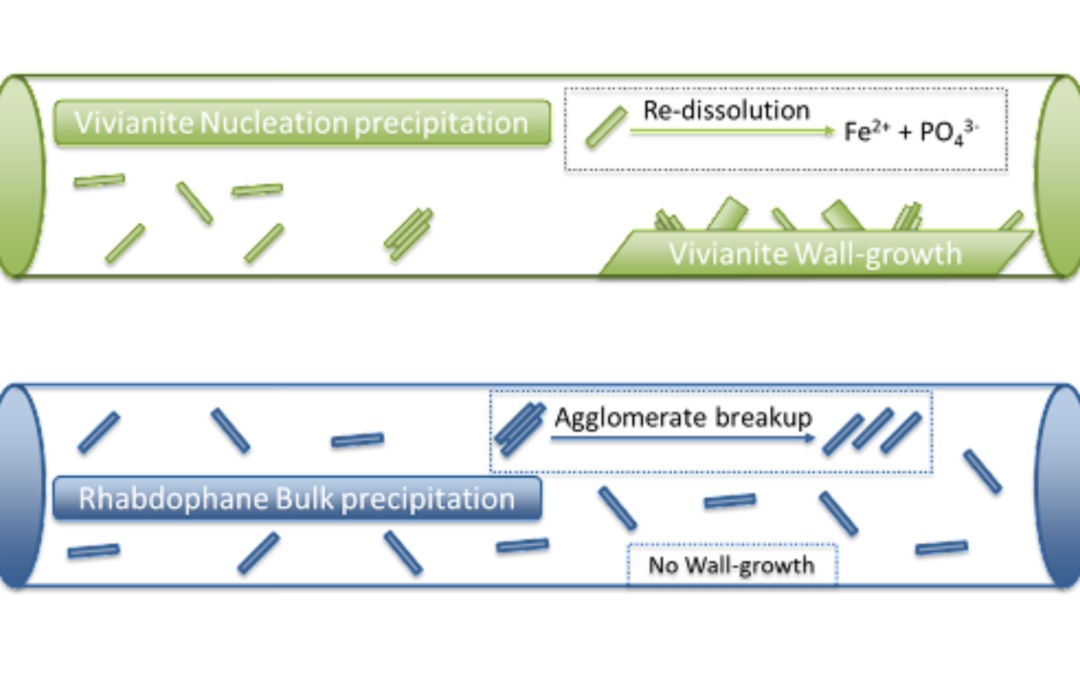
Vivianite (Fe3(PO4)2۰8H2O) forms when iron reacts with phosphorus. As we have covered in previous blogs, iron has a relatively low attraction for phosphorus, so these crystals tend to redissolve in anaerobic conditions and from variations in pH. At 77oF in distilled water, the solubility of vivianite produces a P concentration of 0.002 mg/L. Add to this the mechanism by which P precipitates, and you can get clogs.
You see, vivianite precipitates on a nucleation site, which is often a rough spot on the wall of a pipe or pump impeller. Once crystals start to grow along this surface, pipe wall or impeller, they continue to precipitate out, forming larger and larger crystals until you have a clog, and THAT is a real mess.
Using WaterFX for phosphorus removal avoids this type of crystal formation and the clogging that goes with it.
The lanthanides, Lanthanum and Cerium, in WaterFX have a couple of distinct advantages when it comes to preventing clogs. First, the bond that lanthanides form with phosphate is MUCH stronger, forming rhabdophane, (LaPO4۰H2O or CePO4۰H2O), which precipitates rapidly via a bulk-mechanism. Due to this very strong bond, the crystals formed are a lot less soluble. How much less? Well, in distilled water at 77oF, instead of a P concentration of 0.002 mg/L as with vivianite, the P concentration with lanthanides is 0.000000003 mg/L. That is a whopping 700,000 times LESS soluble than vivianite. Once these discrete crystals form, they stay formed – no re-dissolving.
Second, since precipitation is through rapid bulk precipitation, the discrete crystals formed do not form strong agglomerates. Put simply, they do not grow ON each other as vivianite does. Though some may stick together for brief periods, these smaller crystals tend to break apart easily into small, dense crystals that don’t adhere to walls or each other, and so don’t form clogs.
No wall growth means no clogging, no down time!
Since the rhabdophane, formed when using WaterFX for phosphorus removal, does not grow along walls and impellers, there is no clogging. No clogging means no downtime, no changing out equipment, and no operational headaches. You can learn more about both vivianite and rhabdophane and Neo WaterFX’s no-clog advantage here.