
In addition to being a superior method for phosphorus removal from wastewater, Neo WaterFX helps prevent algal blooms that impact waterways.
How is phosphorus removed from wastewater most reliably, efficiently and cost effectively?
To protect downstream surface waters from eutrophication contributing to potentially toxic algal blooms, regulatory agencies are issuing more discharge permits to wastewater treatment plants (WWTFs) that include increasingly stringent phosphorus limits.
If you operate one of these WWTFs, you are looking for the most cost-effective, reliable, and easy-to-operate system for meeting your phosphorus removal limits.
Biological treatment versus chemical precipitation for phosphorus removal from wastewater
Whether you can adjust your biological treatment process and meet your limits is driven by your influent level, your effluent limit, and your biological treatment system. Influent phosphorus levels range from 5 mg/L to 20 mg/L depending upon your industrial contributions and domestic phosphorus contributions. For domestic only contributions, a moderate level of P is approximately 8 mg/L, with roughly 60% from urine and the remaining 40% from garbage disposals and cleaning products.
Even processes specifically designed for Enhanced Biological Phosphorus Removal (EBPR) through luxury P-uptake are often only capable of a consistently achieving 20% to 60% removal of P. These systems require careful monitoring of the biomass to maximize the population of phosphorus accumulating organisms (PAOs). The oxygen level must be carefully alternated between aerated and anaerobic zones to achieve the maximum uptake of phosphorus. In addition, the oxygen concentration must be maintained in the digesters and clarifiers to prevent release of the phosphorus under anaerobic conditions. Release in the clarifier can result in higher effluent concentrations, potentially violating the permit limits. Release in the digester can result in recycling P back to the headworks with digester decant. EBPR requires a high level of skilled operator attention and a high degree of instrumentation and control to monitor and adjust dissolved oxygen (DO) levels. The process complexity is high and can be subject to operational upsets.
Bottom line, it is unlikely that you will be able to consistently achieve a P effluent limit less than 2 mg/L with biological processes alone, even if you have very low influent P levels. For low P limits, most utilities rely upon chemical precipitation of phosphorus.

Wastewater treatment plant with alternating aeration / anoxic zones for Enhanced Biological Phosphorus Removal (EBPR).
But chemical precipitation agents for phosphorus removal are not created equal.
Traditional drinking water coagulants, ferric and aluminum salts, are also used to lower effluent P levels in wastewater. These traditional salts form a fluffy floc that traps phosphate molecules rather than forming a tight bond. Because these bonds are not tight or selective, these salts require a dosage that is much higher than the 1:1 stoichiometric ratio of salt to P, at least 4 times higher. But for very low limits, P of less than 1 mg/L, that ratio can also easily increase to 8 or more times the stoichiometric requirement.
The high dosage requirement and fluffy floc create a great deal of chemical sludge, a 40% or more increase in volume over the sludge produced without chemical addition. Since dosage ratios increase as the P limit decreases, lower P limits create disproportionately higher increases in sludge volume. This sludge is also difficult to dewater, as the floc traps water resulting in significant bound water that requires high polymer dosages. Sludge dewatering becomes slower, and the final cake will still contain a higher water percentage. The quantity of dewatered sludge requiring disposal increases and with it, the operational cost.
The phosphate precipitates of these traditional coagulants are only insoluble over a narrow range of pH and with moderate dissolved oxygen concentrations. At low DO or with fluctuations in pH, the phosphorus can be released back into the water phase. Release in the clarifier can lead to algae growths on clarifier weirs or even P levels exceeding limits. In the digester, the released phosphorus can be recycled back to the head of the plant with the decant, requiring removal again. Even worse, the P can cause struvite deposits in decant and sludge pumps and piping, requiring costly shutdowns and system maintenance.
These traditional salts are also highly acidic, requiring pH adjustment to achieve the pH required for discharge. This pH adjustment is usually done with caustic, which is highly corrosive and may require heat traced lines. Keep in mind that the higher dosage of acidic coagulant to achieve lower limits will require higher doses of caustic for pH adjustment. Operations are much more complicated, with added safety concerns.

Traditional coagulants used for phosphorus removal in wastewater, like alum, require caustic for pH adjustment and require more polymer for dewatering.
Lanthanide-based coagulants like Neo WaterFX are a better solution for P removal.
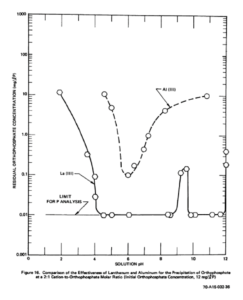
Graph from 1970 study: Phosphate Removal from Wastewaters Using Lanthanum Precipitation, Water Pollution Control Research Series, 1970, 17010EFX 04/70, pg. 33. demonstrating the superiority of lanthanide based coagulants to alum for phosphorus removal in wastewater.
Neo WaterFX’s lanthanide-based coagulants, salts of Lanthanum and Cerium, are selective for phosphate, forming a tight ionic bond at a near 1 to 1 molar ratio. This ratio holds regardless of the concentration or limit, so the 1:1 dosage ratio required remains constant as the limit goes down, rather than increasing to 4:1 or even 10:1 as it does for the traditional coagulants. Not only does this require less chemical, but you know exactly the dosage you need based on your influent level and effluent requirement. Your operators will no longer need to guess about dosage or perform jar tests as your influent level changes.
The precipitate formed is a dense crystal, and although there is a slight increase in chemical sludge, it is a very slight increase, unlike the 40% or more increase from traditional floc-forming coagulants. This precipitate is stable over a broad range of pH and low dissolved oxygen concentrations, eliminating release of P in the clarifier or digester. Your operators will no longer have to scrub weirs or shut down to remove a struvite clogged impeller or pipe. Maintenance is easier and operations are simplified.
Clients (and studies conducted at Virginia Tech) report that the sludge is easier to dewater, requiring less polymer and allowing for shorter run times while producing a drier cake. This saves energy and reduces disposal costs.
With a much higher pH and using 25% to 80% less coagulant to achieve low limits, WaterFX is 100 times less acidic than traditional coagulants. Without the dip in pH, adding caustic for pH adjustment is not necessary. With fewer chemical feeds, plant operation is simpler and safer.
But what are the bottom-line impacts of coagulant choice?
While ferric and alum coagulants are relatively inexpensive chemicals, when you add up the higher dosage requirements, additional sludge, sludge processing, sludge disposal and additional chemical feeds, using ferric or alum coagulants is often much more expensive than the lanthanide-based phosphorus removal. When comparing P-removal coagulants, remember to account for the full O&M cost of operations as well as operator time to monitor and maintain the system. When all cost and operational factors are considered, WaterFX is not only more economical, it is a more reliable solution that improves plant operation.
Want to know more?
If you have a P limit, and are wondering if switching to Neo WaterFX might simplify your operations and reduce your operational expenses, this quick survey will help clarify whether switching makes sense for you.
Or contact us for a pilot study where we can demonstrate Neo WaterFX’s superior phosphate removal capabilities on YOUR wastewater.
Comparison of phosphorus-removal coagulants in wastewater |
||
| Impact | Traditional Fe or Al based | Neo WaterFX lanthanide-based |
| Dosage | Dosage ratio increases dramatically as limits decrease. 4:1 to 8:1 is common depending on P limit. | 1:1 dosage ratio regardless of P limit. |
| Precipitate formed | Loose floc with high water content, slower settling. | Dense crystalline precipitate, rapid settling. |
| Stability of precipitate | Stable over narrow pH range and with moderate DO. May release P in digester or clarifier. | Stable over broad pH range and anaerobic conditions. No release of P in digester or clarifier. |
| Quantity of chemical sludge | 40% more sludge volume is typical, even at relatively high effluent P limits. Lower limits substantially increase sludge volume. | Negligible increase in sludge volume. |
| Dewatering characteristics | Bound water requires higher polymer dosages, longer run times, and producing a wetter cake. | Dense crystals enhance dewatering, requiring less polymer, shorter run times, and producing a drier cake. |
| pH impact | Highly acidic and with high doses required, lowers pH substantially. Requires pH adjustment which increases with dosage. | Much less acidic, and with lower dosage, has minimal impact on pH. No pH adjustment required. |
| Operational impact | Increased complexity with pH neutralization, dewatering impacts, clarifier maintenance, and potential struvite formation. | Simplifies operation with more stable, reliable dosage, fewer chemical feeds, easier more effective dewatering, reduced clarifier maintenance, and elimination of struvite formation.. |


