When it comes to binding with soluble orthophosphate (OP), lanthanide-based coagulants like Neo WaterFX, with cerium chloride (CeCl3) as a main ingredient, have the distinct advantage of being highly efficient and forming a strong bond that holds the phosphate in a crystalline precipitate over a broad range of dissolved oxygen (DO) concentrations and pH levels. This advantage allows wastewater treatment to achieve very low (<0.1 mg/L) limits of total phosphorus (TP) in effluent. But this strong, predictable bonding has advantages in improving the dewatering of digested sludge and preventing struvite formation, which can be essential even without a low phosphorus limit.
Research confirms lanthanide-based cerium chloride superior for struvite prevention and dewatering
Research done by the Virginia Tech Department of Civil and Environmental Engineering, in conjunction with the Upper Occoquan Service Authority (UOSA) of Centreville, Virginia, investigated the mechanisms by which cerium chloride precipitation of OP improved the dewatering of anaerobic digested biosolids. Their research, “Using cerium chloride to control soluble orthophosphate concentration and improve the dewaterability of sludge: Part I. Mechanistic understanding”, was published by Water Environment Research (Water Environment Research• 92:320-330, 2020, https://onlinelibrary.wiley.com/doi/10.1002/wer.1142), along with “Part II. A case study” (Water Environment Research• 92:331-337, 2020, https://onlinelibrary.wiley.com/doi/10.1002/wer.1150). Their research not only confirmed the superiority of cerium chloride for phosphate removal and improved dewatering, but also hypothesized as to the mechanisms responsible for this superior performance. They also determined that using a lanthanide-based precipitate, even seasonally, could save UOSA over $40,000 a year in operational costs.
Phosphorus causing problems even without a limit.
The UOSA WWTF has no phosphorus limit on their discharge permit. But soluble OP was still causing problems, particularly during cold weather months when the utility operates with more reactor zones at very low DO concentrations to promote intensified denitrification. This alternation between low and high DO zones causes increased biological uptake of OP into the biomass. However, in the anaerobic digesters, soluble OP gets released from the biomass due to the lower dissolved oxygen. In addition to being recycled back to the head of the plant with the decant, this phosphate can form a scale inside pipes, valves, and pumps, reducing capacity and increasing maintenance costs. At the UOSA WWTF, it was causing even bigger problems of struvite precipitation in the centrate pump and centrifuge.
The UOSA had been using alum for phosphate removal along with lime for pH adjustment. However, OP removal efficiency is limited by the solubility of the precipitates. The precipitate formed with any of the traditional iron, aluminum, or magnesium-based precipitating agents is much more soluble than that formed with lanthanide salts like cerium chloride. Additionally, phosphate removal with “traditional” coagulants requires overdosing to achieve good removal rates, driving up chemical sludge quantities. These precipitates also produce a gel-like floc that traps water and is difficult to dewater, requiring high doses of polymer to achieve acceptable results.
But even more problematic for UOSA, the alum was not capable of preventing release of OP from the biosolids. In the anaerobic digesters, the reduction in DO causes the biosolids to release the phosphorus, driving soluble OP concentrations over 100 mg P /L, the critical concentration for struvite formation at UOSA. In the centrifuge, where degassing of carbon dioxide raises the pH above 7, this OP combines with ammonia and magnesium to form struvite, leading to maintenance headaches and costly downtime.
Superior precipitation without excessive chemical sludge
The Virginia Tech research team noted that CeCl3 is a more efficient OP precipitate, with high removal efficiency at approximately 1:1 stoichiometric molar ratio of Ce to OP at a neutral pH (no pH adjustment required). They state that the reason for this higher removal efficiency is much lower solubility of the CePO4 precipitate compared to the solubility for any of the “traditional” metal salt precipitates with OP. In addition, CePO4 remains insoluble over a broader range of pH and DO. This means that dosing with CeCl3 can bind with OP as it is being released from the sludge and the insoluble precipitate is formed regardless of pH or DO levels present in the digester.
Because of the 1:1 ratio of Ce to OP removal, the reaction can be controlled with great precision, preventing soluble OP from achieving that 100 mg P/L concentration necessary for struvite formation. This allows UOSA to control the OP concentration at approximately 90 mg P/L without excess chemical feed and with minimal chemical sludge formation, but well under the concentration known to cause struvite formation.
Improved dewatering with cerium chloride results from multiple mechanisms
The evaluations run at Virginia Tech verified that the addition of CeCl3 improved dewatering, allowing for reduced polymer usage while increasing the percent solids in the cake. It should be noted that the evaluation did not include evaluations of sludge from the digester using alum and lime to reduce phosphorus, which would have required dewatering additional chemical sludge with poor dewatering properties.
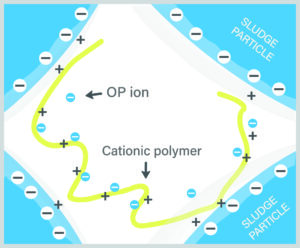
Figure 1: Negatively charged OP ions bind with cationic polymer, hindering polymer bonding with sludge particles and trapping water between sludge particles, making dewatering less effective.
However, the research team presents many interesting ideas as to the mechanisms of this dewatering improvement. They theorize that negatively charged soluble OP in the sludge water phase may occupy binding sites on positively charged polymer, thus competing with negatively charged sludge particles and hindering the polymer from adequately binding with the sludge particles. Figure 1 illustrates this mechanism, showing how OP can attract the cationic bonding sites, pulling the polymer away from the negatively charged sludge particles. This leaves more space between sludge particles
where water becomes trapped, bound between the sludge particles and is difficult to remove. Additional polymer must be used to override this OP binding effect and properly bind the sludge for dewatering, driving up polymer dosage and hindering dewatering efficiency.
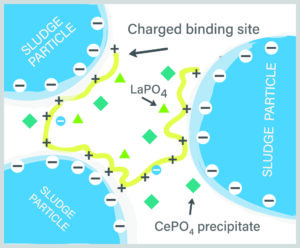
Figure 2: Ce and La bind with OP- to form dense crystal. Binding OP- allows the cationic bonding sites on the polymer to bind with more negatively charged sites on sludge particles, pulling the sludge particles closer together, releasing bound water and facilitating dewatering.
The research team proposed that the improvement seen with the addition of CeCl3 is due to two factors. First, CeCl3 bonds tightly with negatively charged OP ions, taking them out of competition with the sludge for polymer binding sites and facilitating bound water release. Figure 2 illustrates this mechanism, demonstrating how it would work with WaterFX, which is a blend of Cerium (Ce) and Lanthanum (La) salts. The OP– bonds tightly with the Ce or La, forming a dense crystalline precipitate and freeing up cationic bonding sites on the polymer. The polymer then forms multiple bonds with the sludge particles, pulling the sludge particles together and reducing the interstitial space while releasing bound water. The result is a denser sludge requiring less polymer for excellent dewatering. Second, the CePO4 (and LaPO4) formed may create skeleton builders that enhance sludge filterability, release intracellular water, and reduce sludge compressibility. Bottom line: dewatering is easier, faster, and requires less polymer with CeCl3.
Neo WaterFX brings you all the advantages of CeCl3
Whether your goal is meeting a low total phosphorus effluent limit or preventing struvite formation, Neo WaterFX, with CeCl3, will deliver reliable, controllable results while also improving your sludge dewatering. Whatever your phosphorus related concern, you owe it to yourself and your utility to get a free consult to see if WaterFX is the right solution to your phosphorus challenge. Contact us today!



