Achieving low total phosphorus limits in wastewater (under 1 mg/L) requires chemical removal with coagulants. But one problem with traditional iron or aluminum coagulants is that they do not PREFER bonding with phosphate. They prefer bonding with hydroxide OH– ions, forming what Metcalf and Eddy refer to as a “bulky, gelatinous floc” (Metcalf and Eddy, 4th Edition, 2003, p 496). Much of the phosphate removal comes from phosphate molecules being trapped in this gel as it settles, not from actual bonding. So, in addition to forming a gel that traps water and is difficult to dewater and consumes most of the natural alkalinity in your wastewater, these “traditional” coagulants are extremely inefficient at removing phosphorus.
What is not tightly bonded is easily released.
Keeping the phosphorus bound is also hard for these traditional coagulants. Changes in pH or dissolved oxygen can shift the ion equilibrium, breaking up the precipitate flocs and releasing everything that is bound in them. This can occur in your clarifier where the phosphorus can leave in your effluent, potentially causing regulatory violations. More often this occurs in your digester, causing you to recycle the phosphorus back to the headworks in your decant. There you get to remove it all over again along with the “new” phosphorus in your influent.
Neo WaterFX preferentially bonds with phosphate, forming a strong bond.
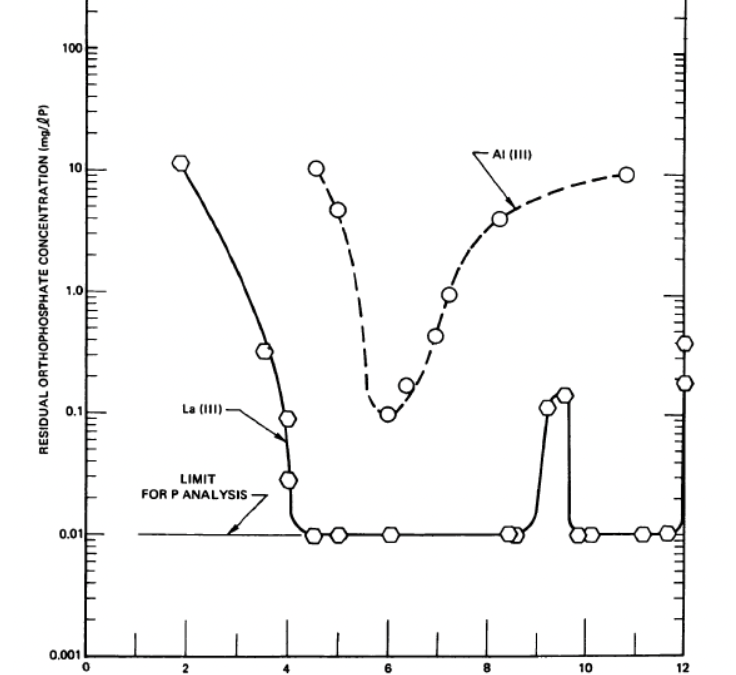
WaterFX uses lanthanide salts, Lanthanum and Cerium, to tightly bond the phosphate to the lanthanide to form a dense precipitate. The result is a bond that holds over a broad range of conditions, particularly pH. As this graphic shows, lanthanum not only removes phosphorus to below 0.01 mg/L (detection limit in 1970) but holds the phosphorus better over a broad range of pH.
Source: Phosphate Removal from Wastewaters Using Lanthanum Precipitation, Water Pollution Control Research Series, 1970, 17010EFX 04/70, pg. 33.
The publication goes on to conclude (pgs. 43-44): “Lanthanum exhibits a considerably broader effective pH range for phosphate removal than observed with aluminum: this is particularly true in the case of polyphosphates. For example, at 2:1 equivalence ratios, practically no removal of pyro- and tripolyphosphates was observed with aluminum at pH levels ± units from that for optimum phosphate removal (pH ~ 5.5). In the case of tripolyphosphate precipitation with lanthanum, the residual phosphate concentration was less than 0.1 mg/L P between pH’s of 6 and 10 and was less than 1.0 mg/L P in the 4 to 10.5 pH range. One very attractive feature of the use of lanthanum for the removal of phosphates from wastewater is that the pH of almost all domestic wastewaters lies within the pH range where lanthanum is most effective.”
Bind and hold, that is the key
In summary, lanthanide salts show superior binding with phosphates over a broad range of pH, and they remain bound even as the pH changes. The result is more reliable P removal, less concern over pH fluctuations, less impact to your alkalinity, and a more readily dewaterable sludge.
Phosphorus Removal From Wastewater
Learn more about Phosphorus Removal From Wastewater.
To learn more about the lanthanide advantages of WaterFX, or to see if WaterFX is right for you, contact our engineers for a free consultation.