Attraction and Bonding: What molar ratio really means for your phosphorus treatment. And why you should care.
First, we get it. When someone mentions molar ratio, the vast majority of people, including wastewater treatment operators, grimace. Their eyes glaze over as their minds flash back to that horrendous chemistry class they had to take. For the minority who were in class that day — proud nerds who really enjoyed chemistry and still can’t get enough of it — you may be interested in this in-depth technical explanation of the molar ratios of various coagulants that remove phosphorus from wastewater systems, and why Neo WaterFX300 (formerly RE300) is superior for achieving low P concentration in your effluent: https://neowatertreatment.com/wp-content/uploads/2017/09/RE_Technology_Chemistry_in_Wastewater.pdf.
For the rest of us mere mortals, molar ratio boils down to the question of how many atoms/molecules of the coagulant do you need to add to reliably remove one molecule of phosphate (PO4). Just like the old joke of how many chemistry teachers does it take to change a lightbulb, fewer is better.
What does it take to benefit from a low molar ratio with your coagulants, and how do different coagulants stack up? To understand that, it helps to examine molecular attraction and bonding types, and how those impact the molar ratio in your treatment process.
See the molar advantage of Neo WaterFX300 (formerly RE300).
Molecular Attraction: Who is Phosphate’s Favorite Valentine?
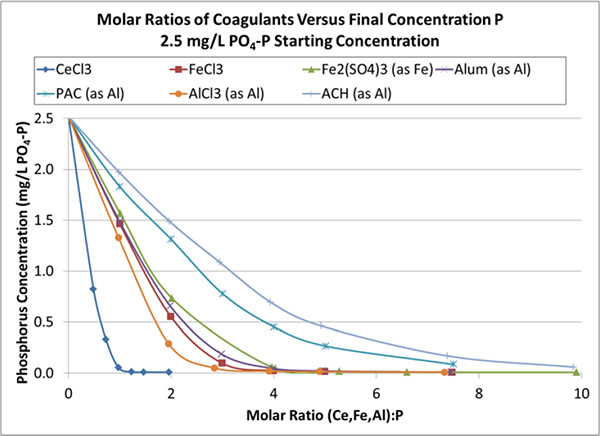
For traditional ferric (Fe) or aluminum (Al) based coagulants, the attraction between PO4 and Fe or Al is not that strong. For starters, Fe or Al form bonds with oxygen and hydroxyl (OH) molecules first, forming intermediate molecules. These molecules clump together to form a flock that then removes PO4 through adsorption. The attraction between the flock isn’t that strong or preferential for PO4, so the adsorbed PO4 can easily be displaced by another molecule. Flocks can adsorb or trap all types of molecules in addition to PO4, including water. This produces a fluffy sludge, with a high sludge volume index (SVI) and a lot of bound water, making it more difficult to dewater. It also requires as much as 3 to 7 atoms of ferric or aluminum to trap just one PO4 molecule and remove it from the system, especially as the concentration of phosphate gets lower. If a phosphate molecule bumps into the flock and sticks, fine, but real attraction just isn’t there.
In sharp contrast, atoms of Neo WaterFX300 (formerly RE300) — predominately Lanthanum (La, atomic number 57) and Cerium (Ce, atomic number 58) provide a strong attraction for PO4,and it will attach to them preferentially to other atoms in the wastewater, even at low concentrations of PO4.
Which brings us to the second component of phosphate removal.
Bonding: Now That I’ve Found You, I’m Never Letting Go
With Fe or Al, PO4 is “just a friend,” and not a very good one at that. They will hang out together, but if another molecule comes along, say a carbonate or hydroxyl, the PO4 may get bumped off its adsorption position and drift off to be picked up by another flock, or maybe not. It is easy to see why there must be a lot of flock, a ratio of Fe or AL to PO4 of at least 3 to 1, for most of the PO4 to be adsorbed. To reach low effluent limits of less than 0.1 mg/L P, a high molar ratio of at least 3 and sometimes more than 7 may be required. A lot of flock also requires a high SVI and bound water.
But Neo WaterFX300 (formerly RE300) and PO4 were chemically made for one another. The La or Ce bonds tightly with the PO4; once bound, they won’t let go. In chemistry speak, this means that the solubility product (Ksp) is very low and the equilibrium-free Neo WaterFX300 (formerly RE300) metal concentration is extremely low. ALL rare earth elements want to bond with PO4, and they will keep doing so as long as PO4 is available, until there is no unbound RE metal or PO4 left. The result is extremely low effluent P.
With Strong Bonding Comes Stability
Unlike the flighty bonds formed with Fe or Al flocks, the bonds of PO4 with the Neo WaterFX300 (formerly RE300) are very stable, forming minerals with no bound water. These minerals precipitate easily and produce a sludge with a low sludge volume index that can be dewatered easily. It also means you need much less Neo WaterFX300 (formerly RE300) than competing products. Since each atom of Ce or La seeks out PO4 to develop this strong bond, very low effluent PO4 can be achieved — below 0.1 mg/L — with a ratio of very close to 1 atom of RE metal to 1 PO4 molecule.
This strong bond delivers another advantage in your digester. The PO4 won’t be released as the sludge digests, as is common with Fe or Al based sludges. Haven’t you gotten tired of removing the same PO4 over and over again, as it is recycled back to your treatment system in the digester decant? Neo WaterFX300 (formerly RE300) won’t let go, so you won’t need to worry about that PO4 showing up again in your headworks.
Attraction Plus Bonding = Low Molar Ratio and Many Other Benefits
The 1:1 molar ratio of Neo WaterFX300 (formerly RE300) to PO4, the result of high attraction and strong bonding, brings several additional benefits to your process:
- Lower chemical feed and storage requirements (less gallons per day needed, fewer shipments, smaller storage tanks, less impact on other aspects of your process like pH).
- Lower SVI – which means lower sludge processing and disposal costs, no matter how you process and dispose of your sludge
- Less bound water – easier dewatering
- No recycling of PO4 back to your process with filter water or digester decant
To find out specifically what it can mean for your process, contact us for a free consultation at: Contact us.
Signup for occasional email updates at https://neowatertreatment.com/contact/get-updates/.



