While it may seem that removal of phosphorus from wastewater using lanthanide salts is innovative technology, in truth it is NOT new. Before there was an EPA, the removal of phosphates using lanthanum, La(III), was studied and compared to removal using aluminum, Al(III).
As far back as 1970 – yes, over 50 years ago – the US Department of the Interior tasked the Federal Water Quality Administration with studying methods of phosphorus removal in wastewater. Clearly, the concern over the nutrient load in our nation’s water is also not new. That report, published in the Water Pollution Control Research Series (Phosphorus Removal From Wastewaters Using Lanthanum Precipitation, document 17010EFX 04/07), detailed the reactions, pH ranges, removal efficiency, and settleability of precipitates from using lanthanum and aluminum, while also discussing the limitations of using ferric and calcium salts for phosphorus removal.
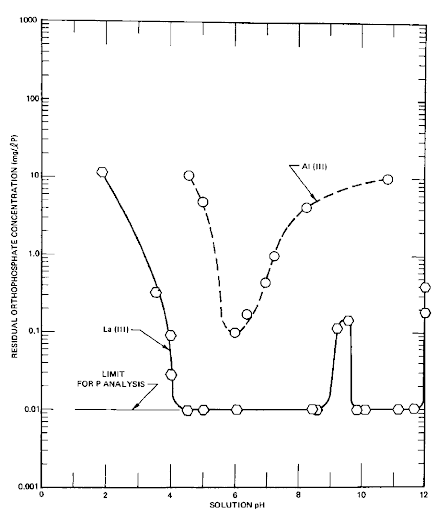
Lanthanum is shown to be “far superior” to aluminum.
The abstract of this report states, “Phosphate removal results indicated that La(III) is far superior for the precipitation of phosphates, especially condensed phosphates, in that it has a broader effective pH range and yields a lower residual phosphate concentration. For example, at a 2:1 cation-to-phosphate equivalence ratio, with phosphates at about 0.001 N, La(III) gave a residual phosphate concentration of less than 0.1 mg/l P for both ortho- and condensed phosphates over a pH range of 6 to 9. With Al(III) this same residual phosphate concentration was obtained only with orthophosphate, and only at a pH of 6.”
As you can see from the above graph of these results, the orthophosphate residual using La(III) within the pH range was often at the (then) limit of analysis, 0.01 mg/l, whereas the Al(III) just barely got to 0.1 mg/l. The abstract goes on to clarify that using La(III) does NOT require a 2:1 ratio, stating “at constant pH, the removal of orthophosphate was found to be directly proportional to the concentration of added lanthanum. Essentially complete removal of phosphate was observed at a La(III)/PO4-3 molar ratio of slightly less than 0.9.” The report concluded that this indicates a purely chemical reaction as the removal mechanism as opposed to adsorption. This chemical reaction brings with it many advantages including predictability, stability of operation, and a very stable, settleable chemical precipitate in much less quantity than precipitates using other coagulants.
Many benefits of lanthanum precipitation are backed up by solid research.
This report goes on to outline many of the advantages of using lanthanum salts over aluminum, including:
- Lower P residuals across multiple types of phosphate.
- No need for pH adjustment to be in the range of optimal removal.
- Since no acid (or excess aluminum salts) are needed to lower pH to the removal zone, destroying wastewater’s buffering alkalinity in the process, no counter pH adjustment using caustic is needed.
- Predictable removal based on stoichiometric chemical reaction even in changing wastewater conditions.
Find the complete report at Phosphate Removal From Wastewaters Using Lanthanum Precipitation.
Neo WaterFX harnesses this superior phosphate removal in dozens of plants worldwide: improving compliance, enhancing treatment and saving money.
While this report demonstrates La(III) superiority over Al(III), it doesn’t even get to the best part – your bottom line. Dozens of plants in approximately thirty states, as well as Canada, Mexico, and parts of Europe, have already harnessed the superior P removal power of lanthanide salts using Neo WaterFX and found that WaterFX saved them significant money in operating costs.
Isn’t it time you found out more about this innovative but far from new technology? Contact us for a free, no obligation consultation and find out how WaterFX can work for you.