Introduction
As clean water becomes scarce, the removal of contaminants from wastewater is becoming increasingly important. Nutrients such as phosphorus (P) in the form of phosphate (PO43-) serve as a food source for algae. Recently excessive amounts of P in natural waters have led to large algal blooms which release toxins into the water and deplete dissolved oxygen concentrations. These consequences result in the death of other fresh water species and the contamination of drinking water sources.1 As a result, new regulations are being put into place that greatly restrict the discharge of P into the environment. New wastewater treatment plant discharge permits are requiring ever lower P concentrations in the effluent. These lower permit limits can be difficult to reach with existing technology. Rare Earth (RE) technology has emerged as a viable alternative technology which can help reduce the effluent P concentration through chemical addition alone. RE technology from Neo Chemicals & Oxides (Neo) is a liquid coagulant that contains the rare earth (RE) elements lanthanum (La) and cerium (Ce). This paper is intended to explain the chemistry, application and toxicity of RE in treating wastewater.
Rare Earth Chemistry in Wastewater
The rare earth elements are the set of 15 elements with atomic numbers 57-71 as well as Yttrium(Y, Atomic number: 39). They are located on the lower portion of the periodic table sometimes referred to as the f-block. Until about 60 years ago, not much was known about these elements. The term “rare earth” is actually a misnomer because the elements are not rare. For example, the two RE elements of interest to this paper, La and Ce, are the 26th and 28th most abundant elements in the Earth’s crust, respectively.2 In nature, rare earth elements are found in over 100 minerals with major deposits in India, South Africa, Brazil, Australia, Malaysia, and the USA.2 The rare earths in general and particularly La and Ce form very strong bonds with oxyanions like phosphate and carbonate. The La and Ce bond with phosphate is so favored that common rare earth containing minerals contain rare earth phosphate. This strong bonding is the basis for the use of rare earths as phosphate removal agents. Rare earth based products like those from Neo have been used for phosphate removal from water in multiple industries like aquarium water treatment,3 pool and spa water treatment,4 and lake remediation.5 In wastewater treatment several patents were published circa 1970 describing the use of rare earth salts for precipitation and removal of phosphate.6 All of this demonstrates rare earth based products are effective at removing P in a variety of water treatment applications.
The typical coagulants used in wastewater treatment are iron (Fe) and aluminum (Al) based. Fe based coagulants include ferric chloride, ferrous sulfate, and ferrous chloride among others. Al based coagulants include aluminum sulfate (alum), sodium aluminate, and polyaluminum chloride (PAC). Recently RE technology has emerged as a viable alternative technology for P removal. The principle difference between Fe, Al and RE based products is the mechanism by which they remove phosphate, as shown in Figure 1. There has been some debate about the mechanism of Fe and Al based products. Originally it was thought that Fe and Al formed FePO4 or AlPO4, but recent studies have shown that the mechanism is more complicated. Smith has reported that the mechanism actually consists of a two-step process. A metal oxide such as Al2O3 or Fe2O3 initially forms which is followed by adsorption of phosphate onto the metal oxide surface.7 This mechanism is consistent with the observed need for increasing amounts of Fe or Al at low phosphate concentrations, i.e. the adsorption process is less efficient when there is little phosphate present.
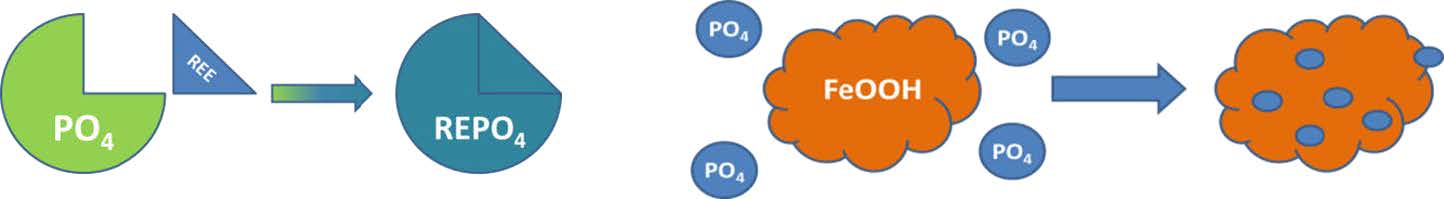
Figure 1. Depiction of phosphate removal reaction mechanism of RE vs. Fe
In contrast, the mechanism for rare earth removal of phosphate is a straightforward metal phosphate precipitation resulting in the formation of the mineral rhabdophane, which is a stable form of RE found in nature. The precipitation reaction can be described by the equation While RE can react in a similar mechanism to that of Fe and Al via the formation of a RE(OH)3 which can adsorb phosphate, the precipitation reaction with phosphate to form rhabdophane is greatly favored.
RE3+ + PO43- -> REPO4·H2O
While RE can react in a similar mechanism to that of Fe and Al via the formation of a RE(OH)3 which can adsorb phosphate, the precipitation reaction with phosphate to form rhabdophane is greatly favored.
Another difference between Fe, Al, and RE based coagulants is the molar ratio of the coagulant metal to P that is needed to remove P to the desired level. The phosphate removal performance of various Fe, Al, and RE based coagulants vs the molar ratio of coagulant to P is shown in Figures 2 and 3. Regardless of starting P concentration, addition of the RE as CeCl3 resulted in the lowest P concentration being achieved when the RE:P ratio was 1:1. By comparison, Fe and Al based coagulants need to be dosed at higher mole ratios (at least 2.5:1 (Fe or Al):P) in order to achieve similar P concentrations.
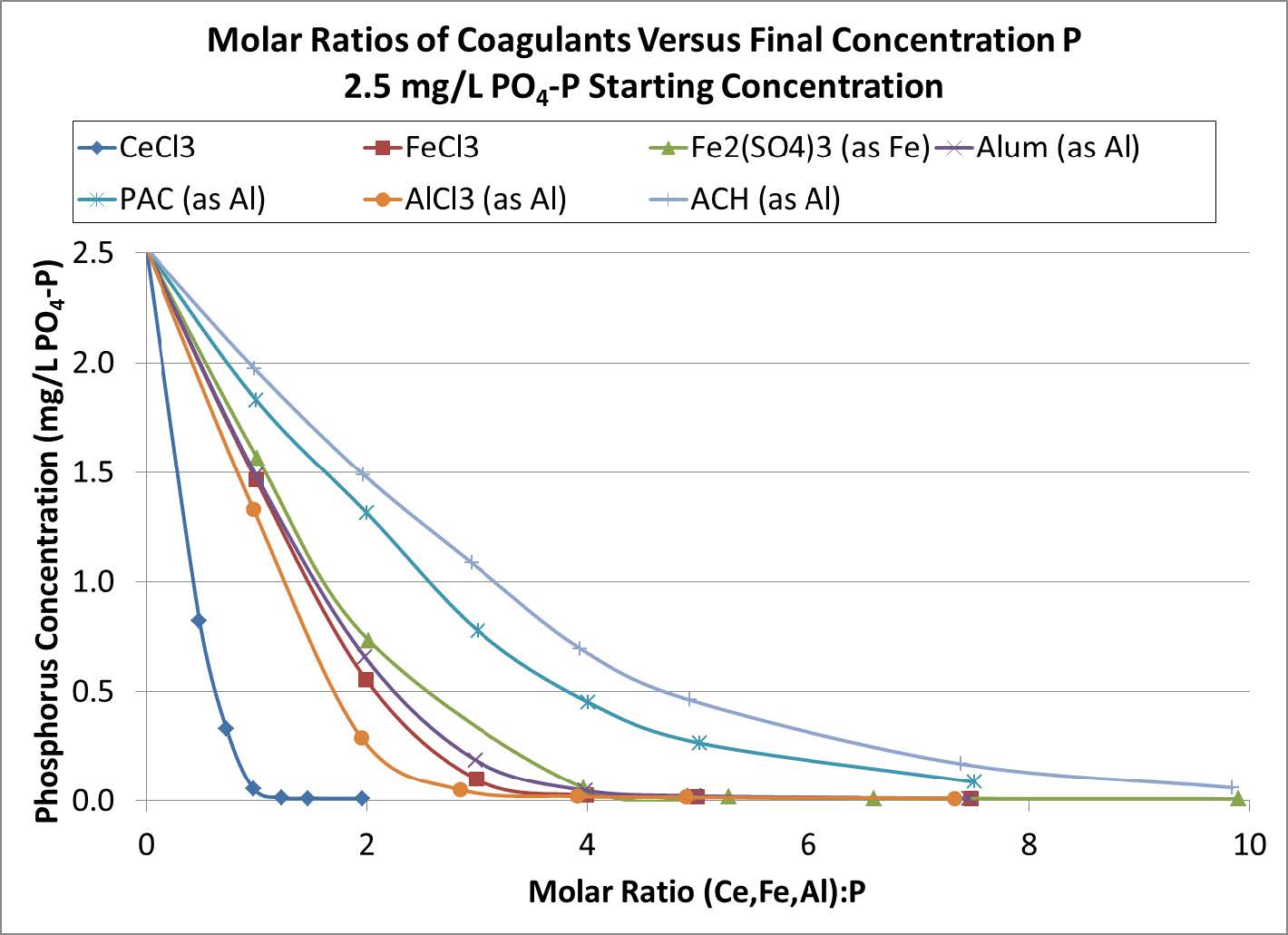
Figure 2. P removal vs. molar ratio for various coagulants. Beginning P concentration of 2.5 mg/L.
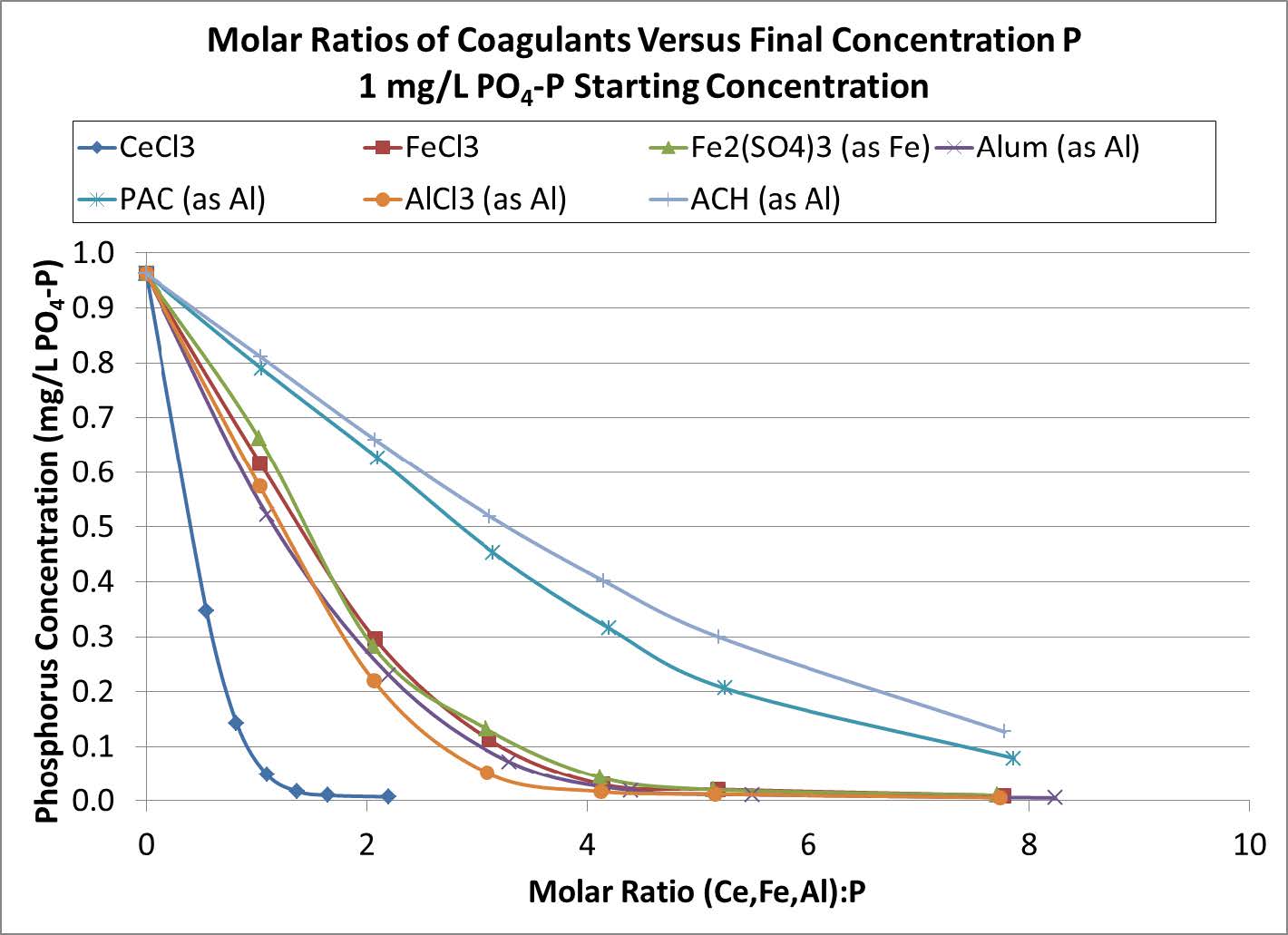
Figure 3. P removal vs. molar ratio for various coagulants. Beginning P concentration of 1.0 mg/L.
The RE to P reaction mechanism accounts for the near 1:1 RE:P molar ratio observed for P removal. In this way RE technology is unique among coagulants.
The addition of RE technology to wastewater has the potential to remove multiple different anions. In addition to P as phosphate, the rare earth elements in RE technology can form insoluble complexes with carbonate (CO32-), hydroxide (OH-), and fluoride (F-). Examples of these reactions are shown in Table I. Also listed in Table I is the precipitation pH range, the solubility product (Ksp) and concentration of RE calculated from the Ksp. The Ksp is a measurement of the solubility of a solid and is calculated by multiplying the equilibrium concentrations of the cation and anion with each raised to the power of its stoichiometric equivalent. For example, for CePO4
sp = [Ce3+][PO43-]
while for CeF3
sp = [Ce3+][F-]3
The lower the equilibrium concentration, the smaller the Ksp, and thus the more insoluble the solid. For practical purposes, the smaller the Ksp is the less soluble the reaction product. As a comparative example, the Ksp of table salt (NaCl) is 3.73×101. This Ksp is 26 orders of magnitude greater than that of cerium phosphate and thus NaCl is very soluble and cerium phosphate is very insoluble.
Table I. Common reactions between rare earths and anions
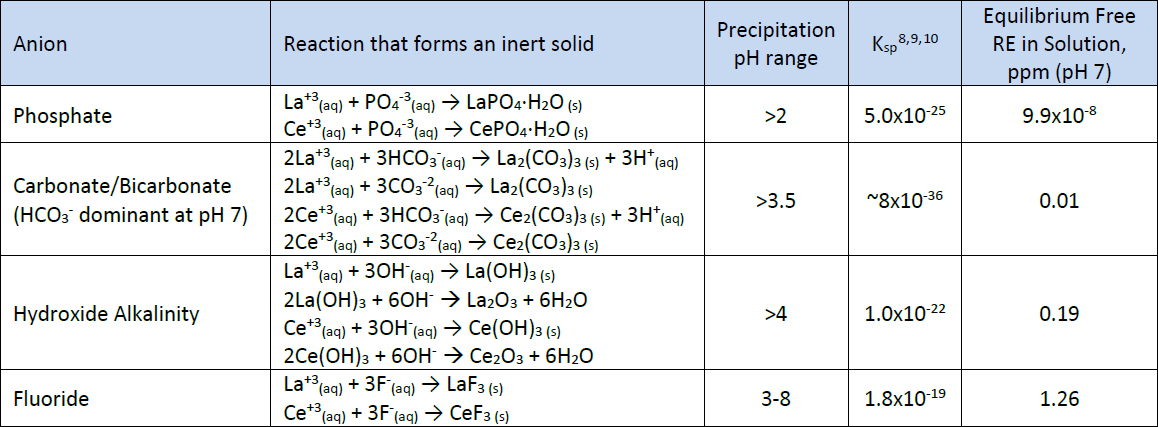
Typical wastewater effluent needs to be in the pH range of 6-9 and as seen in Table I the pH range where RE will precipitate these anions is well within this range. Also the lowest equilibrium free ion concentration calculated from the Ksp in Table I is for the reaction with phosphate. This is further evidence that RE will favor precipitation of P.
Case Studies in Wastewater Treatment Plants
RE technology has recently been used to treat P to meet the low discharge limit of 0.075 mg/L. A 2 MGD wastewater treatment plant discharging into a lake in Minnesota has been evaluating methods to remove P to this level for its upcoming permit. The influent P averages around 2 mg/L but can range from 1-4 mg/L. Initially a trial was done to evaluate the use of a combination of PAC and ferric chloride. While this trial was successful in meeting the discharge limit of 0.075 mg/L, numerous other issues arose related to filtration from the increased dosage of coagulant. Later when RE technology was evaluated, the effluent TP dropped rapidly and within 7 days was below the target limit of 0.075 mg/L (see Figure 4 below).
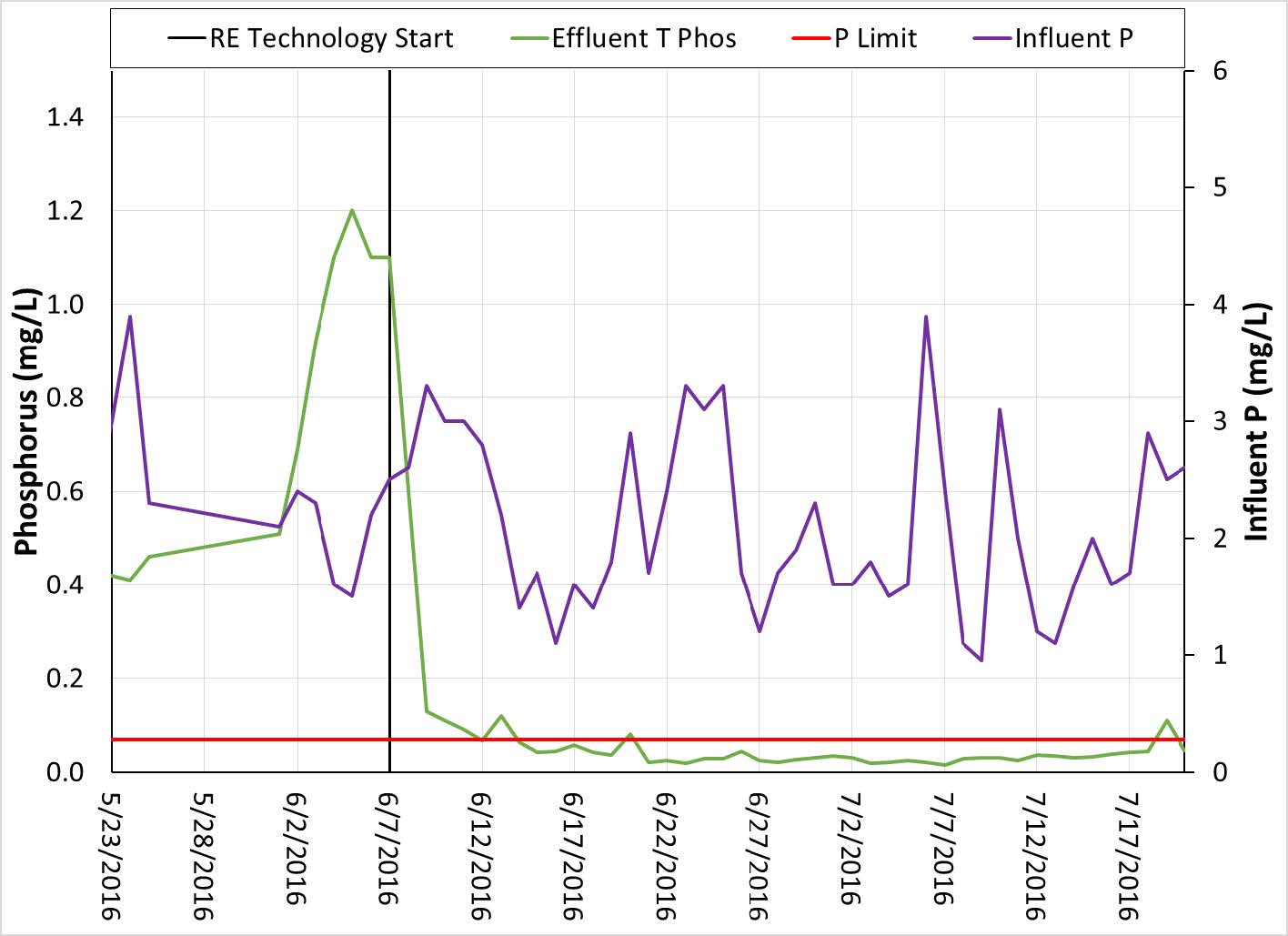
Figure 4. TP discharge at Minnesota Plant using Rare Earth Technology
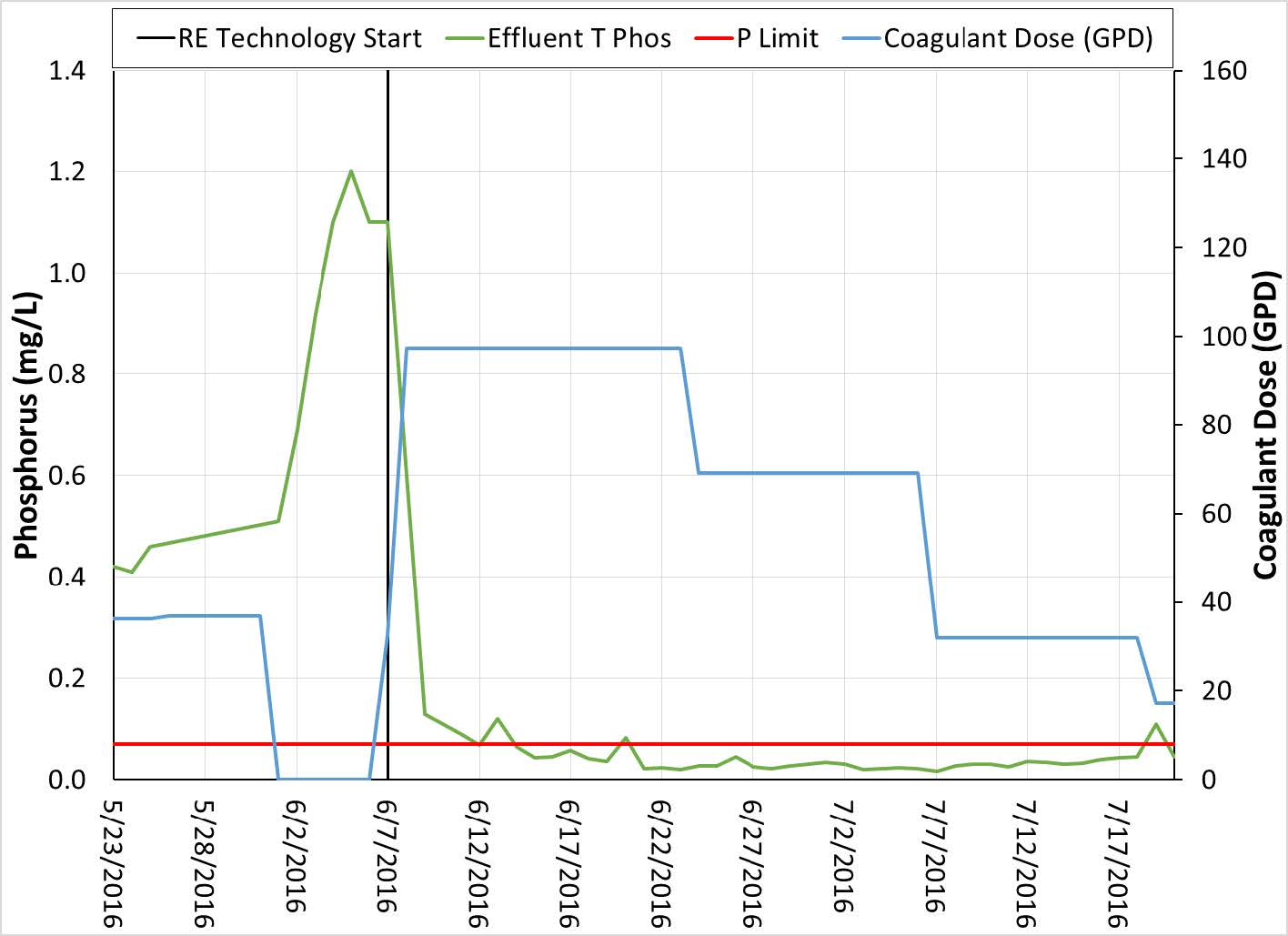
Figure 5. Coagulant dose and TP discharge at Minnesota plant using Rare Earth Technology
The initially high dose rate rapidly gets the RE into the system and can then be lowered while maintaining the TP discharge limit. This method is effective for rapidly lowering the P and optimizing the dose to meet the P demand. When compared to the previous trial using a combination of PAC and ferric chloride, the use of RE technology had several advantages. The first of which was the lack of settling or filtration issues. Secondly the dose rate of coagulant was much lower. The PAC and ferric chloride trial had combined coagulant dose rates of near 180 GPD while RE technology was below 35 GPD.
RE technology has also been used recently in a 3 MGD waste water treatment plant in Wisconsin where the discharge limit for TP is also 0.075 mg/L. Influent TP in this plant varies between 2 and 5 mg/L. Over the course of this trial the influent TP increased slightly. Despite this increase the Effluent TP remained below the discharge limit. As in the trial described above, the initial coagulant dose was high and resulted in a rapid decrease in the effluent TP concentration. The dose was then lowered overtime and the effluent TP remained below the limit.
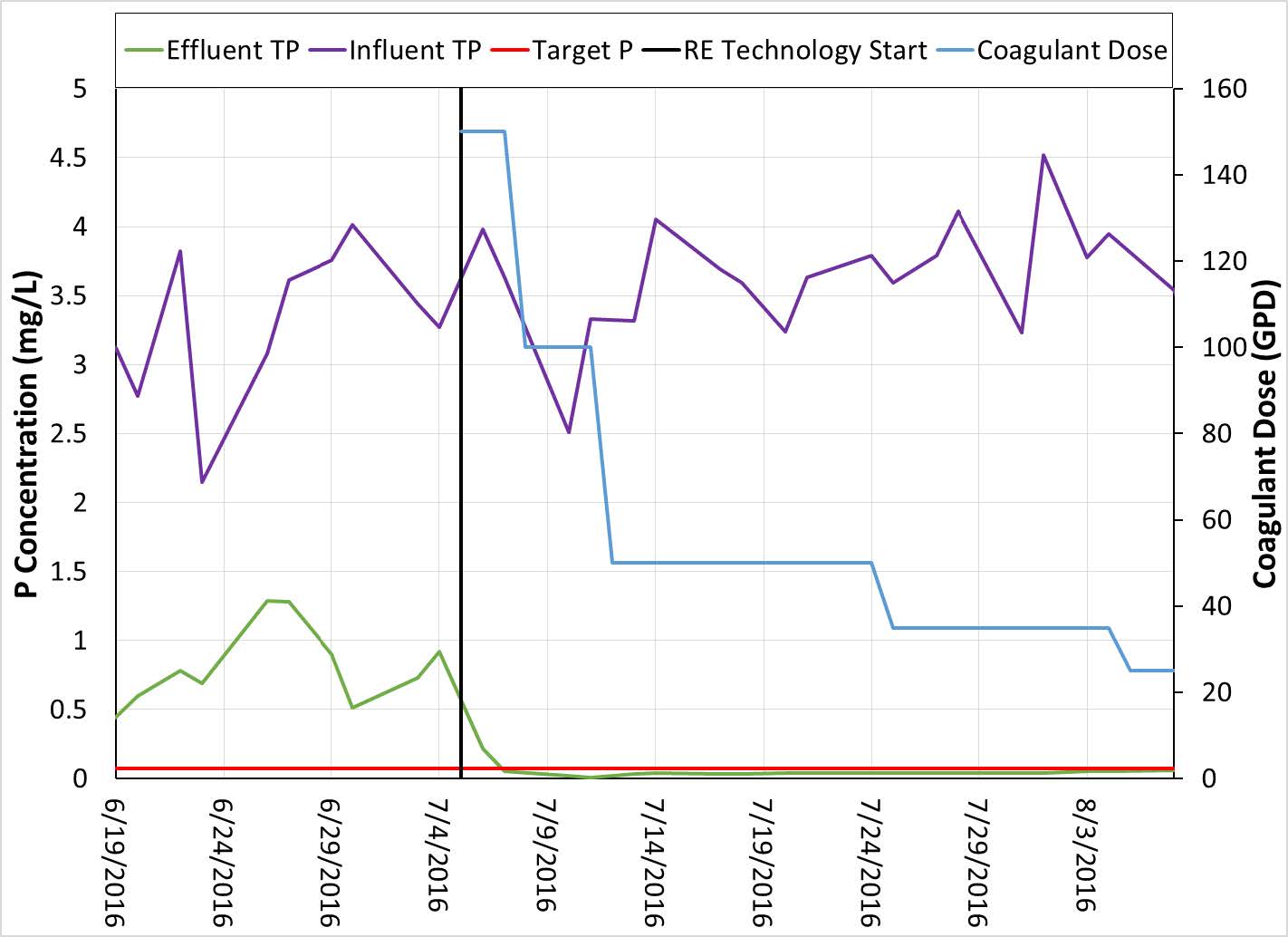
Figure 6. Influent and Effluent TP with Coagulant Dose using RE Technology
Another wastewater treatment plant discharging into a stream that leads to Lake Erie has permits which limit the discharge of Al to 1.1 mg/L and P to 1.0 mg/L. This is a case where the effluent P levels are not extremely low but the amount of Al that can be used is limited. The influent P was in the 3-4 mg/L range. Operators attempted to meet the P discharge limit by alternatively dosing ferric chloride or PAC prior to the primary and secondary clarifiers. Ferric chloride was dosed at high dosage rates and was unable to meet the P discharge limit. Switching to PAC put a limit on the dosing amount because PAC is Al based and would increase the discharge of Al. With RE technology the plant was able to meet both permits using a moderate dose of rare earth. The data from the plant is shown below in Figures 7 and 8. The results show that the addition of RE was more effective at removal of P and had the added benefit of not adding Al to the system.
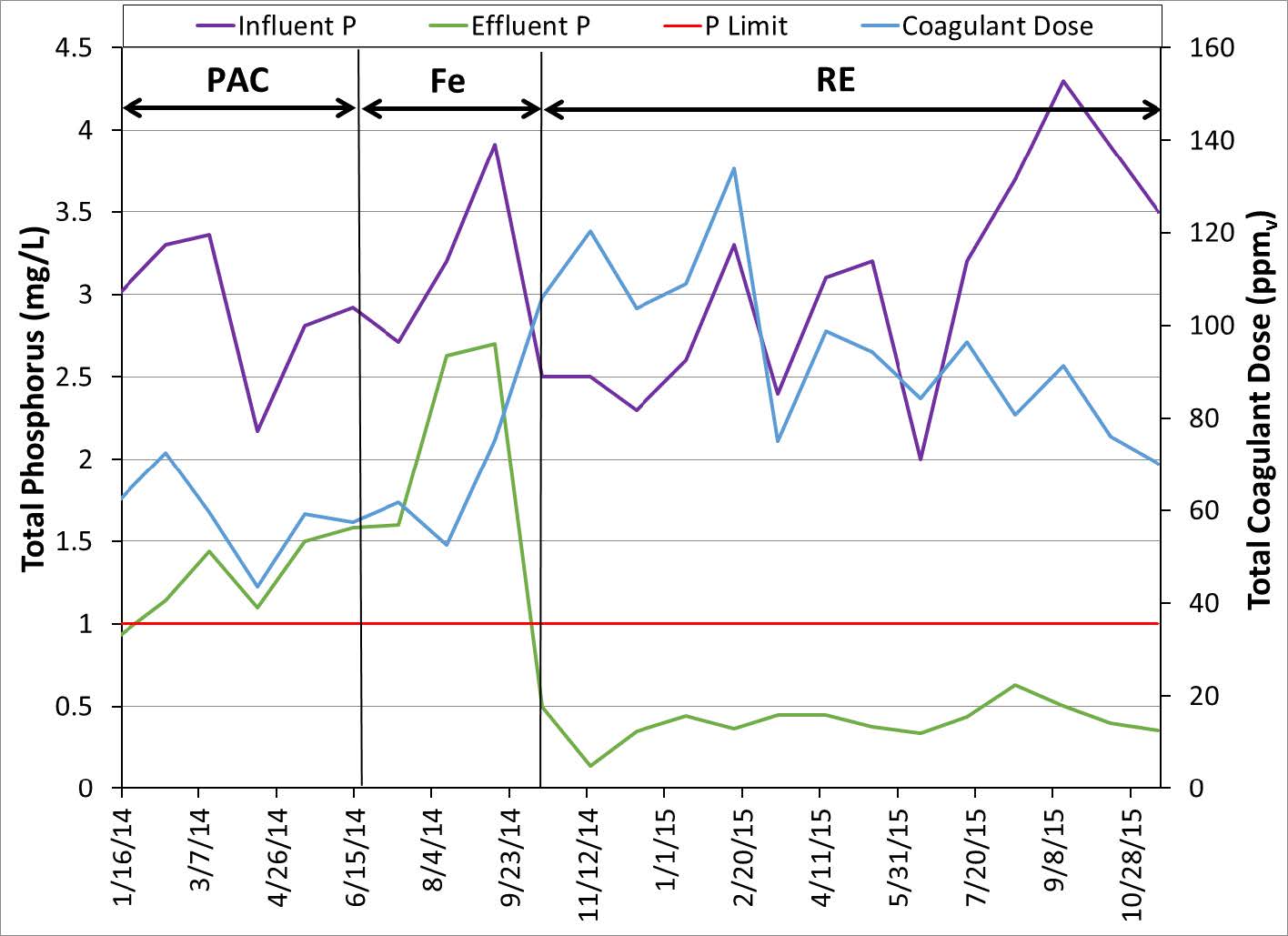
Figure 7. Total Phosphorus before and after RE technology.
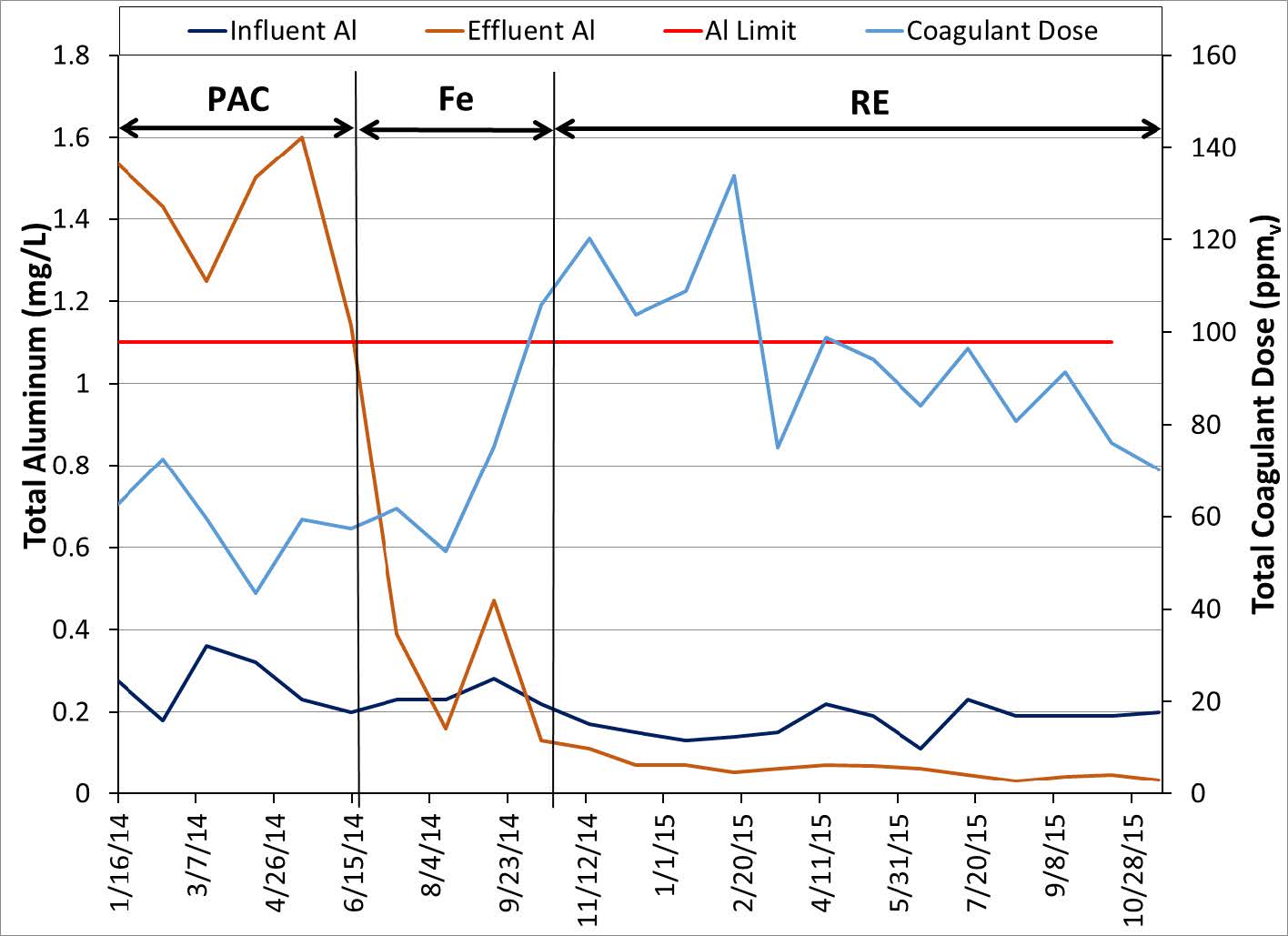
Figure 8. Total Aluminum before and after RE technology.
RE technology has also been used in tertiary treatment. A 30 MGD municipal plant equipped with advanced physical treatment (multi-media filtration and carbon contactors) was using alum to remove P from wastewater. With alum the final effluent P concentration achieved was 0.09 ppm TP. When RE technology was evaluated as an alum replacement, the P concentration achieved was 0.07 ppm TP in the final effluent (see Figure 9 below).
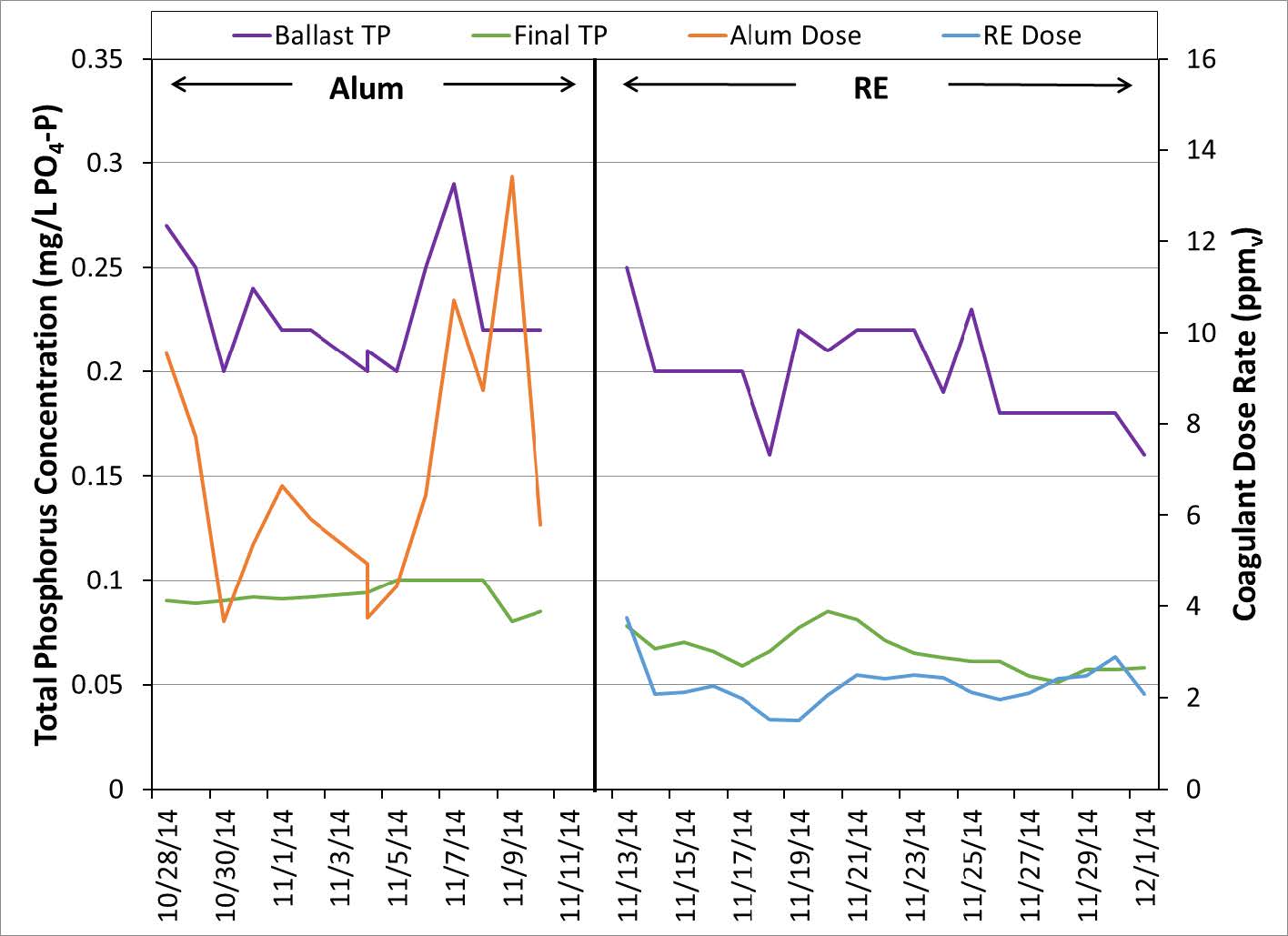
Figure 9. Effluent P concentration before and after RE technology addition
These four examples of the use of RE technology to remove P from wastewater demonstrate the ability to be an effective alternative to the use of typical Fe and Al based coagulants. They show RE technology can be used to achieve very low discharge limits in a variety of plant sizes and dose points.
Toxicity of RE technology in Wastewater
The toxicity of chemical additives to wastewater should be tested to determine the effect of the additives on the ecosystem. Ideally the additive will do what it is designed to do, only be discharged in a minimal amount, and not have any adverse effects on the ecosystem. To confirm this, the EPA has published methods for measuring the toxicity of effluents, the Whole Effluent Toxicity (WET) test.11 Generally the WET test will measure the number of organisms that will survive in water with a dosed amount of either plant effluent or the chemical additive. Freshwater organisms are Ceriodaphnia dubia (daphnid), Daphnia pulex and Daphnia magna (daphnids), Pimephales promelas (fathead minnow), Oncorhynchus mykiss (rainbow trout) and Salvelinus fontinalis (brook trout). The concentration that will kill 50% of the organisms is known as the LC50. Other numbers such as the NOEL (No Observed Effect Level), NOAEL (No Observed Adverse Effect Level), and LOAEL (Lowest Observed Adverse Effect Level) can also be determined from this testing. To date, RE technology has been implemented in over 40 facilities in the USA since 2014. Every facility has passed effluent compliance WET testing while using RE technology.
In an effort to test worst-case toxicity of the additives, it is helpful to assume they are not removed in the treatment process and are discharged directly. LC50 values from published EPA documents and recent Neo studies are shown in Table II below for Ceriodaphnia species and Pimephales promelas. As of this writing, we are unaware of toxicity studies conducted with Fe based coagulants.
Table II. LC50 of Al and RE for Ceriodaphnia and Pimephales species.

The measured LC50 of RE for Ceriodaphnia species is comparable to that of the reported numbers for Al coagulants. This indicates that RE elements have similar toxicity to Al for Ceriodaphnia. However the LC50 of RE for Pimephales promelas is much higher than the reported value for Al. This indicates that REs are less toxic to Pimephales promelas than Al.
The insoluble material generated (sludge) from rare earth addition to wastewater has also been tested. The typical concentration of RECl3 in RE technology is 648 g/L, applied at a volumetric dosage rate of 1:53,000, yields approximately 12 mg REPO44/L. This value is lower than the conservatively calculated safe limit of 14 mg REPO4/L for drinking water.14 The treated water is then further processed through a solids/liquid separation system such as a clarifier or filter. Any rare earth solids that precipitate would therefore be removed, with the actual rare earth phosphate concentration likely to be much lower than 12 mg/L at the point of discharge.15 The primary potential for human exposure to RE technology treated water would be through consumption of treated water, whereas aquatic organisms could be exposed at any point after treatment of a water body with RE technology. Thus little to no harmful effects are expected from solids generated.
Generally rare earths have a low toxicity rating.16,17 There are no known human toxicity studies of cerium phosphate. However, lanthanum carbonate, a commonly used human drug for kidney disease, has been extensively studied.18 In the acidic mammalian gastrointestinal tract, lanthanum carbonate breaks down and combines with phosphate to form lanthanum phosphate, thus ultimately removing the phosphate from the human body. This process is similar to the way RE technology removes phosphate from water bodies. Therefore, the extensive human and animal studies of lanthanum carbonate also address the final lanthanum product in the human body, namely lanthanum phosphate.
Lanthanum carbonate, and therefore lanthanum phosphate, has very low toxicity in humans and animals. There is no indication that lanthanum phosphate is genotoxic (causes cancer or other significant health problems).19 In a bioassay to determine potential genotoxicity, the major component of RE technology, cerium chloride, was found to rapidly form cerium phosphate in the biological system and, like lanthanum phosphate, was not genotoxic. Given the similar chemistry and environmental behavior of lanthanum phosphate and cerium phosphate, there is no reason to believe that cerium phosphate would have different toxicity and should be considered non-toxic for humans at the low concentrations likely to be encountered in water treated with RE technology.
The toxicity of the sludge generated towards denitrification bacteria has also been studied in unpublished sludge respiration inhibition studies. The rare earth solids present in the sludge would be those discussed earlier such as REPO4, RE2(CO3)3, and RE(OH)3. A study conducted by Intrinsic Technologies for Neo found no toxicity towards activated sludge microbes. As a comparison, for CeO2 the EC50 for inhibition of respiration was greater than 1000 mg/L.20 Based on this the addition of RE to wastewater treatment should have no effect on the microbes.
Neo has also evaluated the effect of land applying the sludge generated in treatment plants using RE based coagulants. This study was performed by Richard Wolkowski, Ph.D. (Extension Soil Scientist at University of Wisconsin-Madison) at Alfisol Soil Management, LLC. In this study, P availability to corn from rare earth biosolids was investigated and compared to P availability from a commercial P fertilizer and ferric biosolids. Corn was chosen for the study as it is the most common crop treated with biosolids and is grown on four million acres in Wisconsin. Rare earth biosolids produced soil with P availability between P fertilizer and ferric biosolids as measured by the change in soil test P. The corn whole-plant dry matter yield either was unaffected by the rare earth biosolids. Thus, the application of rare earth biosolids is not expected to affect the growth and yield of corn when applied at normal rates that supply the corn N requirement.
Conclusion
The use of RE technology, a rare earth based coagulant, to remove P from water is quite effective. The basis for this is the solids formed resemble naturally occurring minerals which have very low solubility. Furthermore these mineral forms have low solubility at the normal pH of wastewater which is pH 6-9. Thus the water treated with RE technology will have low concentrations of RE, and P. Toxicity studies have also shown RE based coagulants and the precipitates formed when used in wastewater to be non-toxic to have low toxicity. Furthermore the presence of RE in the land applied sludge has no observed impact on the growth of corn plants. For these reasons RE based coagulants are a viable option for use in wastewater treatment.
2 Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements, 2nd Ed.; Butterworth-Heinemann: Oxford, 2001.
3 For references regarding rare earth use in aquariums see
-
- Knop, D. New Phosphate Fighter? Coral Magazine, 2009, 6(6), p 2-11.
- Zhang, Y.; Wong, K. S. Lanthanum chloride or lanthanum carboxylate for orthophosphate removal in seawater aquarium – a feasibility study, Environmental laboratory, ZOE Division, Ocean Park, Hong Kong
Yaiullo, J. Featured Aquarium: Atlantis Marine World. Advanced Aquarist. February 2007, Volume VI. (https://www.advancedaquarist.com/2007/2/aquarium)
4 For references regarding rare earth use in pools and spas see
-
- Mills, D. J. Removal of Phosphate from Water. U.S. Patent 6,946,076, Sep. 20, 2005.
- Mills, D. J. Treatment of Swimming Pool Waters. U.S. Patent 6,146,539, Nov. 14 2000.
- Kulperger, R.; Okun, R.; Munford, G. Methods and Compositions Using Lanthanum for Removing Phosphate from Water. U.S. Patent 6,524,487, Feb. 25, 2003.
- Kulperger, R.; Okun, R.; Munford, G. Methods and Compositions Using Lanthanum for Removing Phosphate from Water. U.S. Patent 6,338,800, Jan. 15, 2002.
- Lowry, R. N. Phosphate Removal…Revisited. Pool & Spa Marketing magazine, 2003, 27(7), p 57.
5 For references regarding rare earth use in lake remediation see
-
-
- Peterson, S. A. Nutrient Inactivation as a Lake Restoration Procedure: Laboratory Investigations, U.S. Govt. Print. Off., 1974.
- https://www.sepro.com/phoslock/
-
6 For patents regarding rare earth use in wastewater see
-
-
- Kleber, E. V.; Recht, H. L. Removal of Phosphate from Waste Water. U.S. Patent 3,956,118, May 11, 1976.
- Daniels, S. L.; Parker, D. G. Removal of Phosphate from Waste Water. U.S. Patent 3,617,569, Nov. 2, 1971.
-
7 Hanlon, C. Scott Smith: Understanding and Maximizing Phorphorus Removal INFLUENTS, Fall 2015, p 8-9.
8 Ksp data for REPO4 species can be found in the following references
- Liu, X.; Byrne, R. H. Rare Earth and Yttrium Phosphate Solubilities in Aqueous Solution, Geochimica et Cosmochimica Acta, 1997, 61(8), pp. 1625-1633.
- Firsching F. H.; Brune, N. Solubility Products of the Trivalent Rare-Earth Phosphates Chem. Eng. Data 1991, 36, pp. 93-95.
- Cetiner, Z.S.; Wood, S.A.; Gammons, T.C. H. The aqueous geochemistry of the rare earth elements. Part XIV. The solubility of rare earth element phosphates from 23 to 150°C. Chemical Geology 2005, 217 pp. 147– 169. (and references there in)
9 Ksp data for RE2(CO3)3, RE(OH)3, and REF3 species can be found in the following references
- Spahiu, K.; Bruno, J. A Selected Thermodynamic Database for REE to be used in HLNW Performance assessment Exercises, MBT Tecnologia Ambiental, 1995.
- Baes, C. F.; Mesmer, R. E. The Hydrolysis of Cations, Wiley, 1976.
- Firsching, F. H.; Mohammadzadei, J. Solubility Products of the Rare-Earth Carbonates, J. Chem. Eng. Data 1986, 31(1), pp. 40-42.
10 Menon, M. P.; James, J.; Jackson, J. D. Complexation, Solubilities and Thermodynamic Functions for Cerium(III) Fluoride-Water System, Journal of Radioanalytical and Nuclear Chemistry 1986, 102(2), 419-428.
11 Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, Fifth Ed. October 2002. U.S. Environmental Protection Agency EPA-821-R-02-012.
12 Ambient Aquatic Life Water Quality For Aluminum- 1988 EPA-440/5-86-008
13 Unpublished data from Neo Chemicals & Oxides on RE 300 and converted to a RE basis. LC50 for ceriodaphnia dubia is 48 hr and for pimephales promelas is 96 hr.
14 The oral reference dose for the similar compound Lanthanum Phosphate is 0.5 mg/kg-day (NSF International 2010 as cited by United States Environmental Protection Agency (USEPA). 2012. Rare earth elements: A review of production, processing, recycling, and associated environmental issues. EPA/600/R-12/5721. August.). Thus the safe daily exposure level of a rare earth phosphate is 0.5 mg/kg-day * 70 kg = 35 mg/day (assuming a 70 kg person). Using the 80% upper ceiling (United States Environmental Protection Agency (USEPA). 2000. Methodology for deriving ambient water quality criteria for the protection of human health. Office of Water, EPA-822-B-00-004. October. Available online: https://water.epa.gov/scitech/swguidance/standards/upload/2005_05_06_criteria_humanhealth_method_complete.pdf) contaminant exposure from water the safe concentration is 0.5 mg/kg-day * 70 kg * 0.8/(2 L/day consumed) = 14 mg/L.
15 REPO4 will be detected in a total phosphorus test. Assuming all 12 mg/L REPO4 was discharged, the TP measured would be 1.6 mg P/L, which is much higher than new discharge permits coming into effect (<1 or <0.1).
16 Kilbourn, B. T. Cerium: A Guide to Its Role in Chemical Technology. Molycorp, Inc.: Fairfield, NJ, 1992.
17 Haley, T. J. Pharmacology and Toxicology of the Rare Earth Elements. J. Pharm. Sc, 1965, 54, 663
18 For references see
- Food and Drug Administration (FDA). 2004. Review and Evaluation of New Toxicology Data Submitted with Fosrenol® NDA Resubmission (NDA 21-468). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-468_Fosrenol_Pharmr.pdf
- Hutchison, A.J., et al. 2009. Lanthanum carbonate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease Stage 5 receiving hemodialysis. Clin Nephrol. 71:286-95.
- Finn, W.F., et al. 2005. A long-term, open-label extension study on the safety of treatment with lanthanum carbonate, a new phosphate binder, in patients receiving hemodialysis. Curr Med Res Opin. 21:657-64.
19 ANOHSC (Australian National Occupational Health and Safety Commission). 1998. Available online: https://www.nicnas.gov.au/__data/assets/word_doc/0015/20175/NA606FR.docx.
20 Toxicological Review of Nano Cerium Oxide. Prospect 2010




